Soil Fertility and pH: The Foundation of Productive Farmland
By halderman
Fertility is often thought of as the amount of specific nutrients in soil, e.g., phosphorus, potassium, nitrogen, etc. However, since the interest in these nutrients is almost always motivated by a desire to grow crops as productively as possible, there are a few other factors that are closely related and can be even more important than soil test nutrient levels. One of these factors is pH.
Halderman Farm Management works hard to maximize the productivity of every farmland asset we steward. Soil tests are a key component to monitoring the soil health and fertility and pH is one measure we always focus on.
pH stands for “potential Hydrogen”. The scientific explanation is that pH. is specifically the inverse log of the Hydrogen ion concentration on a scale from 0-14. That definition is a little challenging for most of us who aren’t chemists to conceptualize. The easier way to think about it for most of us is as a measure of acidity. A pH of 7 is neutral, neither acidic nor alkaline. Anything below 7 is an acidic soil and anything above is alkaline.
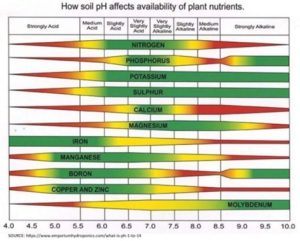
The traditional and most often mentioned reason that soil pH is important is that the soil pH determines how available the nutrients in the soil are to plants. This is primarily because the pH affects the chemical form of nutrients and consequently their availability to plants. The chart below shows the impact of soil pH on the availability of a few of the nutrients plants require. The target pH can vary with the crop produced, but for the typical mix of crops grown on midwestern agricultural soils (corn, beans, wheat, alfalfa), the most efficient pH level is slightly acidic, around 6.5. Targeting a range of 6.2 to 6.8 is a reasonable range and one that can maintain soil productivity well.
Another important aspect of pH is the impact on the microbial population in soil. Both bacteria and fungi are necessary life forms for a healthy soil. Bacteria prefer a neutral to slightly alkaline soil, pH’s of 6.5 to 8 are where they are most active. Fungi can have a wider range of tolerance to pH but they will make up an increasingly larger share of the microbial life of the soil as pH moves lower below 6. Even within these broad microbial groups, there are differences in pH tolerance between the thousands of microbial species in the soils. The best target range for supporting the desired diversity of soil life is considered to be 6.0 – 7.5. Since microbial activity is a key part of the soil processes that drive conversion to plant-available forms of several nutrients, it should not be surprising that the optimal pH range for nutrient availability and microbial activity are very similar.
One of the factors affecting soil pH is parent material. Different geographic areas deal with different challenges with natural pH levels. Parent material, climate, and organic matter content, as well as the management and crops grown, can all impact soil pH and whether it tends toward acidic or alkaline. For example, the use of ammonium-based nitrogen fertilizer, which is very common in corn production, will tend to lower pH, i.e., acidify the soil over time.
When soil tends to become more acidic than desired under agricultural production, the situation can usually be remedied by the application of lime. Calcitic lime is primarily comprised of calcium carbonate. Soil moisture will slowly break down the lime, and the calcium will be released as positive ions and the carbonate as negative ions. The carbonate will react with positive hydrogen ions (the presence of which defines the acidity of the soil) in a way that will reduce the free hydrogen and thereby raise pH. Dolomitic lime includes magnesium carbonate in addition to calcium carbonate, and the same process occurs with both compounds with regard to reducing free hydrogen and raising pH.
When pH is alkaline (above 7) elemental sulfur is the most commonly used to reduce pH and increase soil acidity. However, in many cases with alkaline soils it is impractical or cost-prohibitive to lower soil pH significantly. Good management of higher pH soils will include considering the alkaline nature of the soil when selecting herbicides. This is important because one of the impacts of high pH in agricultural production is on both the efficacy of many herbicides and also the potential to negatively impact the following year’s crop because the herbicide does not break down in the soil as fast as would typically be expected. Adding organic matter, e.g., manure, can have an acidifying effect on soils over time. Another method of managing alkaline soils when it may be impractical to try to lower pH throughout a field, is planter application of sulfur products that can somewhat lower pH in the root zone of plants.
Soil pH is far too important to be ignored in any fertility management program. It is often considered to be the most important aspect of soil fertility since in impacts the availability of both plant nutrients and the microbial life that defines soil health.
If you have questions about the soil fertility on your farm please reach out to Halderman at 800-424-2324 or www.halderman.com.
